Atomic Structure - Line Spectra:
GO BACK
Bunsen and Line Spectra

The colors of fireworks are familiar to all of us and
serve as a wonderful place to begin in understanding the work of Robert Bunsen.
A brilliant page describing the physics of fireworks including both black body
and atomic emissions may be viewed by clicking here.
Bunsen began his career doing organic chemistry, but after an explosion that
cost him an eye and several weeks of illness, he found it prudent to find
another area of research. When Bunsen joined the faculty at Heidelberg he had
gas brought into his laboratory. In order to produce a flame with l see soot he
developed the burner that goes by his name today.
Bunsen's work examined the spectra of elements in flames.
It was well known that many salts cause flames to assume brilliant colors. The
set below are representative of these lovely colors.
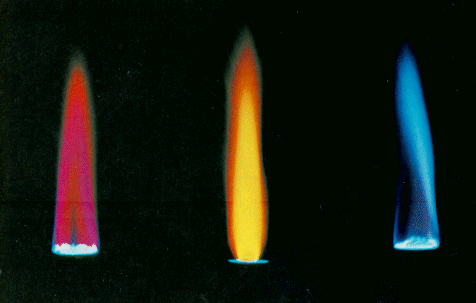
In order to examine these flames, Bunsen used a simple
apparatus involving a couple of slits, a prism, and a telescope (later replaced
by a strip of photographic film). With this device, Bunsen was able to establish
that these salts emitted only certain lines of the spectrum and not a continuum
of colors as you'd expect from a black body. Below are illustrated the
difference between the spectral out put of a hot body and of a number of common
elements:

Notice that the common elements in the flame emit only
specific wavelengths of light, and that every element has a highly
characteristic pattern of light emission.
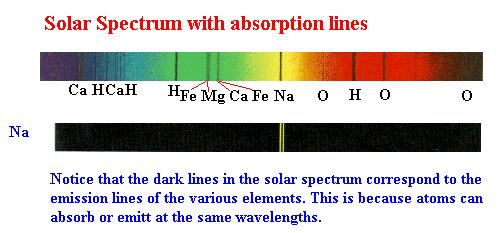
When high resolution techniques became available to
examine the spectrum of the sun, it was realized that the sun's emitted light
was a continuum as would be expected for a very hot object, but that there were
dark lines in this spectrum caused by the absorption of light by elements in the
gaseous corona surrounding the sun. In the illustration below, several of these
lines are identified. Notice, it is the presence of these heavy elements in the
sun that provides strong evidence that the sun must be a second or third
generation star.
In 1885, a Swiss schoolteacher (woops guys wrong
nationality and name) Johann Balmer examined the line spectrum of hydrogen and
found that it fit the simple equation:
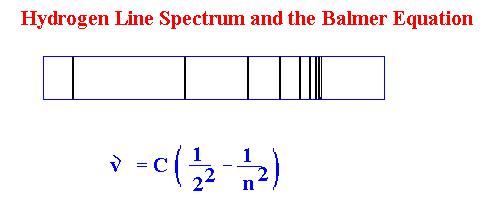
Needless to say this is an odd equation and did little to
clear up the confusion about the origins of the line spectra. Making life even
more complex, while this equation worked for hydrogen, it was not useful for any
other atoms.
 We've already mentioned
the German physicist Max Planck (1858 - 1947), but it is time to return to this
brilliant scientist. Planck had joined the faculty of The University of Berlin
and begun to study black body radiation. The light emitted from a black body was
well studied at that time and several workers had tried to develop an equation
for the curve. Applying highly sophisticated thermodynamics, Planck was able to
develop an equation for the curve, but the physical interpretation of the
equation eluded him. While equations may seem at first to just be jumbles of
symbols, to a mathematician or physicist (and some chemists, too) they are like
sentences to be read as if telling a story. For Planck it was like having a
sentence written in another language. In the process of developing a theoretical
basis for his equation Planck realized it was necessary to introduce energy as
small packets rather than as a continuously variable quantity. As the idea of
these energy packets, or quanta, violated the rules of physics as they were then
understood and Planck struggled with breaking the rules. In 1931 an American
physicist asked Planck how he had invented something as outlandish as the
quantum theory. Planck replied "It was an act of desperation. For six
years I had struggled with the blackbody theory. I knew the problem was
fundamental and I knew the answer. I had to find a theoretical explanation at
any cost, except for the inviolability of the two laws of thermodynamics." We've already mentioned
the German physicist Max Planck (1858 - 1947), but it is time to return to this
brilliant scientist. Planck had joined the faculty of The University of Berlin
and begun to study black body radiation. The light emitted from a black body was
well studied at that time and several workers had tried to develop an equation
for the curve. Applying highly sophisticated thermodynamics, Planck was able to
develop an equation for the curve, but the physical interpretation of the
equation eluded him. While equations may seem at first to just be jumbles of
symbols, to a mathematician or physicist (and some chemists, too) they are like
sentences to be read as if telling a story. For Planck it was like having a
sentence written in another language. In the process of developing a theoretical
basis for his equation Planck realized it was necessary to introduce energy as
small packets rather than as a continuously variable quantity. As the idea of
these energy packets, or quanta, violated the rules of physics as they were then
understood and Planck struggled with breaking the rules. In 1931 an American
physicist asked Planck how he had invented something as outlandish as the
quantum theory. Planck replied "It was an act of desperation. For six
years I had struggled with the blackbody theory. I knew the problem was
fundamental and I knew the answer. I had to find a theoretical explanation at
any cost, except for the inviolability of the two laws of thermodynamics."
Planck's paper titled "Uber des Gesetz der
Energieverteilung im Normalspectrtum", "On the Law of Energy
Distribution in Normal Spectra", Annalen der Physik, 4,
1901, 553.
As a consequence of his quantum ideas Planck established
that the energy of light should be proportional to its frequency by the
equation:
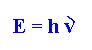
The constant "h" in this equation is a
fundamental unit of quantum energy and is now called Planck's constant.
 While Planck was
struggling with the concept of the quantum, Albert Einstein (1879 - 1955) was
muddling along in school. Einstein did not like the rigid teaching techniques of
his German teachers and frequently came into conflict with them. His work in
secondary school was undistinguished and when he applied to the Polytechnical
School in Zurich he was refused admission. After study in a school roughly
equivalent to a modern community college, he was accepted to the Polytechnical
School. Even though he had had to put in extra effort to get into the
Polytechnic, Einstein showed no real potential and was little noted by his
teachers. Upon graduation friends found him a position as a patent clerk in
Berne, Switzerland, a job that gave him considerable time to sit and think and
write. While Planck was
struggling with the concept of the quantum, Albert Einstein (1879 - 1955) was
muddling along in school. Einstein did not like the rigid teaching techniques of
his German teachers and frequently came into conflict with them. His work in
secondary school was undistinguished and when he applied to the Polytechnical
School in Zurich he was refused admission. After study in a school roughly
equivalent to a modern community college, he was accepted to the Polytechnical
School. Even though he had had to put in extra effort to get into the
Polytechnic, Einstein showed no real potential and was little noted by his
teachers. Upon graduation friends found him a position as a patent clerk in
Berne, Switzerland, a job that gave him considerable time to sit and think and
write.
The results of that thought appeared in a series of three
papers in Annalen der Physik in 1905. One of these papers "Uber eine die
Erzeugung und Verwandlung des Lichtes betreffenden heuristischen Gesichtspunkt",
"Heuristic viewpoint concerning the generation and transformation of
light"( Annalen der Physik, 17, 1905, 132.) built upon the
work of Planck, and proposed that light itself was quantized and consisted of
"particles of energy hv"! Einstein states in this paper "Despite
the strangeness of this result, certain consequences derived from it are in
perfect harmony with the observed facts." The implication that light was a
particle that displayed wave properties was later described as the duality of
light and has become an article of faith in modern science.
 Nowhere did the seed of
quanta find more fertile ground than the mind of Neils Bohr (1885 - 1962). Bohr
was raised in a middle class Danish family and showed no particular talent as a
child except for sports. He played soccer at almost a professional level and was
an active skier until late in his life. Bohr studied physics as an undergraduate
at the University of Copenhagen and, upon graduation, traveled to Cambridge to
study under J. J. Thompson. Thompson was particularly busy at this point and was
not able to work with Bohr so Bohr took up an invitation from Rutherford to
travel to Manchester and study radioactivity (one presumes with his soccer ball
under his arm). Nowhere did the seed of
quanta find more fertile ground than the mind of Neils Bohr (1885 - 1962). Bohr
was raised in a middle class Danish family and showed no particular talent as a
child except for sports. He played soccer at almost a professional level and was
an active skier until late in his life. Bohr studied physics as an undergraduate
at the University of Copenhagen and, upon graduation, traveled to Cambridge to
study under J. J. Thompson. Thompson was particularly busy at this point and was
not able to work with Bohr so Bohr took up an invitation from Rutherford to
travel to Manchester and study radioactivity (one presumes with his soccer ball
under his arm).
With Rutherford, Bohr participated in some of the studies
on alpha particle scattering by heavy metals, and began to examine the planetary
ideas of the atom that had been proposed and rejected by Rutherford. Simply put,
if one imagined an atom in which the negatively charged particles orbited the
positive nucleus, one ran into a critical physical problem. So long as particles
travel in straight lines they may retain their momentum, but when they curve in
their path they must lose energy. Modern high energy light sources such as the National
Light Source in Brookhaven, NY take advantage of this phenomenon by having
beams of particles travel through magnetic fields where the particles must
change direction and give off energy in the form of light. What this meant was
that if an electron was to travel in a circle around a nucleus, it must lose
energy and slowly spiral into the nucleus. (There was actually a science fiction
story published in the 1920's around the theme of a shrinking universe based on
this idea.)
Bohr was very familiar with the new ideas developed by
Planck and Einstein and wrote up a preliminary proposal to describe a quantum
atom in June of 1912 which he was going to discuss with Rutherford. On talking
to a friend he mentioned this talking paper and the friend asked whether Bohr's
ideas could account for the spectrum of hydrogen. Curiously, Bohr hadn't
considered this question and the friend recommended that Bohr look at Balmers'
equation to see if the model fit the data. Bohr later related "As soon as I
saw Balmers' formula the whole thing was immediately clear to me".
Bohr's final model of the planetary atom was published in
the Philosophical Magazine in 1913. In those days a paper to such a journal had
to have a senior sponsor and Rutherford forwarded the contribution of his young
colleague despite its "wild ideas". Bohr's atomic model built upon a
set of postulates:
- Electrons exist in stable orbits about the nucleus.
- Only certain orbits are allowed
- An electron passing from a lower orbit to a higher
orbit must absorb light with exactly the difference in energy between the
two orbits. Similarly, for an electron to move from a higher orbit to a
lower one, it must emit light corresponding to exactly the difference in
energy.
Using the appropriate constants Bohr was able to derive
the Balmer equation and exactly predict the spectrum for hydrogen. Of particular
importance, Bohr's equation predicted additional spectral lines for hydrogen
which were rapidly sought out and confirmed by experimental workers. Since
prediction is one requirement of a successful scientific theory, Bohr's theory
appeared to be off to a bright future.
Bohr's model allowed a simple understanding of the absorption
or emission of
light by atoms that explained line spectra. The hot buttons lead you to figures
on these topics.
It is relatively simple to picture the mechanics of Bohr's
model so it has become the starting point for students in chemistry.
Unfortunately, Bohr's model was quickly found to be flawed. It only really
worked for hydrogen and could not be modified to fit anything beyond lithium.
The model was useless in describing bonding, and it relied on a set of arbitrary
postulates whose justification flew in the face of known physics. At best,
Bohr's model must be seen as a transitional theory, introducing the quantum
theory to the understanding of atoms, but not a fully modern theory.
The next step along the way awaited a young man who
originally began his studies as a historian before he turned to the dark side.
Original source for this page: http://www.chem.uidaho.edu/~honors/spectra.html
Last modified
Contact us for more info

| 