Avogadro's Hypothesis:
Equal volumes of all gases measured at the
same temperature and pressure contain the same number of molecules. (Since
flask 1 & flask 2 are the same size, and the same temperature and pressure,
then there must be the same number of molecules of Gas A and Gas B.)
If Gas A in flask 1 weights 5.50 grams, and
Gas B in flask 2 weighs 2.0 grams, what is the weight of one molecule of Gas A
compared to one molecule of Gas B?

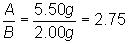
So, one molecule of Gas A is 2.75 times as
heavy as one molecule of Gas B.
Last modified
Contact us for more info

| 