Heat of Fusion:
Heat of fusion is the amount of heat required to melt 1
mole of a solid at its melting point.
1) Calculate the amount of heat necessary to melt
36.0 g of water. (heat of fusion of H2O = 1.44
kcal/mol)
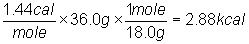
2) Calculate the heat of fusion of sodium chloride,
given the data that 1.16 g of NaCl absorbed 135 cal of heat in changing from a
solid to a liquid.
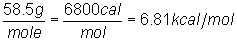
The same amount of heat is given off when a liquid
solidifies, as is absorbed when the solid melts.
3) Calculate the amount of heat given off as 10.0 g of
liquid sodium solidify. (Heat of fusion of Na = 0.63 kcal/mole)

Last modified
Contact us for more info

| 