Molality - Boiling Point Elevation:
In dilute solutions, the elevation of the boiling point is
directly proportional to the number of solute molecules (or moles) in a given
weight of solvent. The effect of a nonelectrolyte dissolved in water can
be calculated by:
kb x m = DTb
(kb = molal B.P. constant for H2O
= 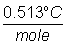 ) )
(m = molality of the
solution)
Then, the boiling point of the solution can
be calculated by: Boiling point of solvent + DTb
Example:
What is the boiling temperature of a solution containing
30.0 g of a nonelectrolyte (MM = 180 g/mol) dissolved in 100 g (0.100 kg) of
water?
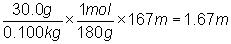
kb x m = 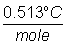 x
167 m = 0.857 oC
= DTb x
167 m = 0.857 oC
= DTb
B.P. of water + DTb
= 100.000 oC (at 1 atm) + 0.857 =
100.857 oC
Last modified
Contact us for more info

| 