Stoichiometry (Rules):
Rules for determining amount of substance
reacting or formed in chemical equations:
-
Balance the equation.
-
Decide what quantity of each
substance is given. Write it below the substance (one line
down if a mole quantity, two lines down if a laboratory
quantity).
-
Decide what quantity you want
to calculate. Represent it with a question mark directly
under the substance (one line down if a mole quantity, two lines
down if a laboratory quantity).
-
Complete the diagram with
arrows by going from the known laboratory quantity to moles,
then to moles of unknown quantity, and finally to laboratory
quantity of the unknown. (Step(s) can be omitted if either
or both the given quantity and the unknown quantity are moles.)
-
Calculate each quantity
following the steps in the diagram.
Click Here for an example
How many liters (at STP) of NH3
are needed to react with 80.0 g of O2?
1)
4 NH3
+ 5 O2 -->
4 NO + 6 H2O
(step 2)
2)
moles  moles
moles
(step 3)
 (step 1) (step 1)
? L
80.0 g
3) 80.0 g O2 x 1 mole / 32.0
g = 2.5 moles O2 (step 1)
From the
balanced equation: 5 moles O2 consumed 4 moles of NH3
so, 2.5 moles O2 will consume 2 moles NH3
(step 2)
2 moles NH3 x 22.4 liters
(STP) / 1 mole = 44.8 liters NH3 (STP)
PowerPoint
Chemical Reactions of Copper and Percent
Yield
Objective: To gain familiarity
with basic laboratory procedures, some chemistry of a typical transition
element, and the concept of percent yield.
Apparatus and Chemicals:
|
0.5 g piece
of no. 16 or no. 18 copper wire |
evaporating
dish |
|
250 mL beaker
(2) |
weighing
paper |
|
concentrated
HNO3 |
6.0 M H2SO4 |
|
graduated
cyliner |
granular zinc |
|
3.0 M NaOH |
methanol |
|
carborundum
boiling chips |
acetone |
|
stirring rod |
towel |
|
iron ring and
ring stand |
balance |
|
wire gauze |
aluminum foil
cut in 1-inch squares |
|
Bunsen burner |
concentrated
HCl |
Discussion:
Most chemical synthesis involve separation
and purification of the desired product from unwanted side products. Some
methods of separation, such as filtration, sedimentation, decantation,
extraction, and sublimation were discussed earlier. This experiment is
designed as a quantitative evaluation of your individual laboratory skills in
carrying out some of these operations. As the same time you will become
more acquainted with two fundamental types of chemical reactions -- redox
reactions and metathesis (double-displacement) reactions. By means of
these reactions, you will finally recover the copper sample with maximum
efficiency. The chemical reactions involved are the following.
Cu(s) + 4 HNO3(aq)
-----> Cu(NO3)2(aq) + 2 NO2(g)
+ 2 H2O(l)
Redox
[1]
Cu(NO3)2(aq) +
2 NaOH(aq) -----> Cu(OH)2(s)
+ 2 NaNO3(aq)
Metathesis [2]
Cu(OH)2(s) ----->
CuO(s) + H2O(g)
Dehydration [3]
CuO(s) +
H2SO4(aq) -----> CuSO4(aq)
+ H2O(l)
Metathesis [4]
CuSO4(aq)
+ Zn(s) -----> ZnSO4(aq)
+ Cu(s)
Redox [5]
Each of these
reactions proceeds to completion. Metathesis reactions proceed to
completion whenever one of the components is removed from the solution, such as
in the formation of a gas or an insoluble precipitate (driving forces).
This is the case for reaction [1], [2], and [3], where in reactions [1] and [3]
a gas and in reaction [2] an insoluble precipitate are formed. Reaction
[5] proceeds to completion because zinc has a lower ionization energy or
oxidation potential that copper.
The objective in
this experiment is to recover all of the copper you begin with in analytically
pure form. This is the test of your laboratory skills.
The percent yield of
the copper can be expressed as the ratio of the recovered weight to initial
weight, multiplied by 100:
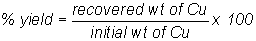
Procedure:
-
Weight approximately 0.500 g
of no. 16 or no. 18 copper wire (1) to the nearest 0.0001 g and
place it in a 250 mL beaker. Add 4-5 mL of concentrated
HNO3 to the beaker. IN THE HOOD. After the
reaction is complete, ass 100 mL distilled H2O.
Describe the reaction (6) as to color change, evolution of gas,
and change in temperature (exothermic or endothermic) in the
report sheet.
-
Add 30 mL of 3.0 M
NaOH to the solution in your beaker and describe the reaction
(7). Add two or three boiling chips and carefully heat the
solution -- while stirring with a glass stirring rod -- just to
the boiling point. Describe the reaction on your report
sheet (8). Remove the boiling chips.
-
Allow the black CuO to
settle; then decant the supernantant liquid. Add about 200
mL of very hot distilled water, stir, and then allow the CuO to
settle. Decant once more. What are you removing by
washing and decanting (9)?
-
Add 15 mL of 6.0 M H2SO4.
What copper compound is present in the beaker now (10)?
Your instructor will tell you whether you
should use zinc or aluminum for the reduction of Cu (II) in the following step.
A. Zinc
In the hood, add 2.0 g of 30-mesh zinc metal
all at once and stir until the supernatant liquid is colorless. Describe
the reaction on your report sheet (11). What is present in solution (12)?
When gas evolution has become very slow, heat the solution gently (but do
not boil) and allow it to cool. What gas is formed in this reaction (13)?
How do you know (14)?
B. Aluminum
In the hood, add several 1-inch squares of
aluminum foil and a few drops of concentrated HCl. Continue to add pieces
of aluminum until the supernatant liquid is colorless. Describe the
reaction on your report sheet (11). What is present in solution (12)?
What gas is formed in this reaction (13)? How do you know (14)?
When gas evolution
has ceased, decant the solution and transfer the precipitate to a preweighed
porcelain evaporating dish (3). Wash the precipitated copper with about 5
mL of distilled water, allow to settle, decant the solution, and repeat the
process. What are you removing by washing (15)? Wash the precipitate with
about 5 mL of methanol (KEEP THE METHANOL AWAY FROM FLAMES _ IT IS FLAMMABLE!)
Allow the precipitate to settle, and decant the methanol. (METHANOL IS
ALSO EXTREMELY TOXIC: AVOID BREATHING THE VAPORS AS MUCH AS POSSIBLE.)
Finally, wash the precipitate with about 5 mL of acetone (KEEP THE ACETONE AWAY
FROM FLAMES - IT IS EXTREMELY FLAMMABLE!), allow the precipitate to settle, and
decant the acetone from the precipitate. Prepare a steam bath as
illustrated and dry the product on your steam bath for at least 5 minutes.
Wipe the bottom of the evaporating dish with
a towel, remove the boiling chips and weigh the evaporating dish plus copper
(2). Calculate the final a\weight of copper (4). Compare the weight
with your initial weight and calculate the percent yield (5). What color
is your copper sample (16)? Is it uniform in appearance (17)?
Suggest possible sources of error in this experiment (18).
Pre-lab (Review Questions):
1. Give an example, other than the ones
listed in this experiment, of redox and metathesis reactions.
2. When will reactions proceed to
completion?
3. Define percent yield in general
terms.
4. Name six methods of separating
materials.
5. Give criteria in terms of
temperature changes for exothermic and endothermic reactions.
6. If 1.65 g of Cu(NO3)2
are obtained from allowing 0.93 g of Cu to react with excess HNO3,
what is the percent yield of the reaction?
7. What is the maximum percent yield in
any reaction?
8. What is meant by the terms
decantation and filtration?
9. When Cu(OH)2(s) is
heated, Copper (II) oxide and water are formed. Write a balanced equation
for the reaction.
10. When sulfuric acid and copper (II)
oxide are allowed to react, copper (II) sulfate and water are formed.
Write a balanced equation for this reaction.
11. When copper (II) sulfate and
aluminum are allowed to react, aluminum sulfate and copper are formed.
What kind of reaction is this? Write a balanced equation for this
reaction.
REPORT SHEET: Chemical Reactions of
Copper and Percent Yield
1. Weight copper initial
_______________
2. Weight of copper and evaporating
dish
_______________
3. Weight of evaporating dish
_______________
4. Weight of copper final
_______________
5. % Yield (show calculations)
_______________
6. Describe the reaction Cu(s)
+ HNO3(aq) -->
7. Describe the reaction Cu(NO3)2(aq)
+ NaOH(aq) -->
8. Describe the reaction Cu(OH)2(s)
-->
9. What are you removing by this
washing?
10. What copper compound is present in
the beaker?
11. Describe the reaction CuSO4(aq)
+ Zn(s), or CuSO4(aq) + Al(s)
12. What is present in solution?
13. What is the gas?
14. How do you know?
15. What are you removing by washing?
16. What color is your copper sample?
17. Is it uniform in appearance?
18. Suggest possible sources of error
in this experiment.
POST LAB QUESTIONS
1. If your percent yield of copper was
greater than 100%, what are two plausible errors you may have made?
2. Consider the combustion of methane,
CH4:
CH4(g) + 2 O2(g)
CO2(g) + 2 H2O(g)
Suppose 2 mole of methane is allowed to react
with 3 mol of oxygen.
a) What is the limiting reagent?
b) How many moles of CO2 can be
made from this mixture? How many grams of CO2?
3. Suppose 8.00 g of CH4 is
allowed to burn in the presence of 6.00 g of oxygen. How much (in grams)
CH4, O2, CO2, and H2O remain after
the reaction is complete?
4. How many milliliters of 6.0 M
H2SO4 are required to react with 0.80 g of CuO according
to Equation [4]?
5. If 2.00 g of Zn is allowed to react
with 1.75 g of CuSO4 according to Equation [5], how many grams of Zn
will remain after the reaction is complete?
6. What is meant by the term limiting
reagent?
Last modified
Contact us for more info

| 