Weak Acids : GO BACK
Weak Acids
– dissociate incompletely (~20%)
Strong
Acids – dissociate completely (~100%)
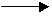 A(g)
+ 2 B(g) A(g)
+ 2 B(g) 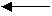 3 C(g) +
D(g)
3 C(g) +
D(g)
Equilibrium constant (Keq)
=
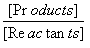 Keq =
Keq =
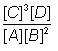 LeChatelier’s
LeChatelier’s
(lu-SHAT-el-YAY’s)
Example Problems:
Pg 521 (black book) & pg 519 (transparency)
IONIZATION
CONSTANTS for
ACIDS
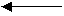
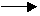 HC2H3O2(
aq)
H+(aq) +
C2H3O21-(aq) HC2H3O2(
aq)
H+(aq) +
C2H3O21-(aq)
(Please
note, in all examples I incorrectly typed C2H5O21-
instead of C2H3O21-)
Equilibrium
constant Keq
=
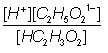 =
Ka =
Acid dissociation constant
=
Ka =
Acid dissociation constant
Ka
= 1.8 x 10-5 @
25 oC
Ka
=
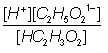
1.8
x 10-5 =
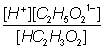
Ka
 HCl H+
+ Cl1-
very large
HCl H+
+ Cl1-
very large
 HNO3
H+ +
NO31-
very large
HNO3
H+ +
NO31-
very large
 H2SO4
H2SO4
 H+ +
HSO41-
large
H+ +
HSO41-
large
 HC2H3O2
H+
+ C2H3O21-
1.8 x 10-5
HC2H3O2
H+
+ C2H3O21-
1.8 x 10-5
 H2S
H+ +
HS1-
9.5 x 10-8
H2S
H+ +
HS1-
9.5 x 10-8
Last modified
Contact us for more info

| 