Development of Periodic Table: GO
BACK
  
Should
space aliens visit earth, I suggest communications begin with a blank periodic
table--anyone capable of space travel would surely recognize the unique shape.
So what's the origin of this universally recognized shape or did Mendeleev
(father of the periodic table) come down a mountain carrying the famous outline
carved in rectangular stone?
The quest for a systematic arrangement of the elements
started with the discovery of individual elements (see Discovery
of Elements). By 1860 about 60 elements were known and a method was
needed for organization. In fact many scientists made significant
contributions that eventually enabled Mendeleev to construct his table. The
periodic table did not end with Mendeleev but continued to take shape for the
next 75 years.

The development of the periodic table begins with German chemist
Johann
Dobereiner (1780-1849) who grouped elements based on similarities.
Calcium (atomic weight 40), strontium (atomic weight 88), and barium (atomic
weight 137) possess similar chemical prepares. Dobereiner noticed the
atomic weight of strontium fell midway between the weights of calcium and
barium:
Ca
Sr Ba (40
+ 137) ÷ 2 = 88
40
88 137
Was this merely a coincidence or did some pattern to the
arrangement of the elements exist? Dobereiner noticed the same pattern for
the alkali metal triad (Li/Na/K) and the halogen triad (Cl/Br/I).
Li Na K
Cl Br I
7
23 39
35 80 127
In 1829 Dobereiner proposed the Law of Triads: Middle element
in the triad had atomic weight that was the average of the other two members.
Soon other scientists found chemical relationships extended beyond triads.
Fluorine was added to Cl/Br/I group; sulfur, oxygen, selenium and tellurium were
grouped into a family; nitrogen, phosphorus, arsenic, antimony, and bismuth were
classified as another group.
First Periodic Table
It was a 19th century geologist who first recognized periodicity
in the physical properties of the elements. Alexandre
Beguyer de Chancourtois (1820-1886), professor of geology at the School of
Mines in Paris, published in 1862 a list of all the known elements. The list was
constructed as a helical graph wrapped around a cylinder--elements with similar
properties occupied positions on the same vertical line of cylinder (the list
also included some ions and compounds). Using geological terms and
published without the diagram, de Chancourtois ideas were completely ignored
until the work of Mendeleev.
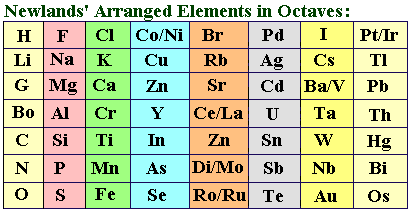
Law of Octaves   
English chemist John
Newlands (1837-1898), having arranged the 62 known elements in order of
increasing atomic weights, noted that after interval of eight elements
similar physical/chemical properties reappeared. Newlands was the first to
formulate the concept of periodicity in the properties of the chemical elements.
In 1863 he wrote a paper
proposing the Law of Octaves: Elements exhibit similar behavior to the eighth
element following it in the table.
Mendeleev's Periodic Table

Then in 1869, Russian chemist Dimitri
Mendeleev (1834-1907) proposed
arranging elements by atomic weights and properties (Lothar
Meyer independently reached similar conclusion but published results after
Mendeleev). Mendeleev's periodic table of 1869 contained 17 columns with
two partial periods of seven elements each (Li-F & Na-Cl) followed by two
nearly complete periods (K-Br & Rb-I).
In 1871 Mendeleev revised the 17-group table with eight
columns (the eighth group consisted of transition elements). This table
exhibited similarities not only in small units such as the triads, but showed
similarities in an entire network of vertical, horizontal, and diagonal
relationships. The table contained gaps but Mendeleev predicted the discovery of
new elements. In 1906, Mendeleev came within one
vote of receiving the Nobel Prize in chemistry. 

 
Noble Gases
 
Lord
Rayleigh (1842-1919) and William
Ramsey (1852-1916) greatly enhanced the periodic table by discovering
the "inert gases." In 1895 Rayleigh reported the discovery of a
new gaseous element named argon.
This element was chemically inert and did not fit any of the known periodic
groups. Ramsey followed by discovering the remainder of the inert gases and
positioning them in the periodic table. So by 1900, the periodic table was
taking shape with elements were arranged by atomic weight. For example,
16g oxygen reacts with 40g calcium, 88g strontium, or 137g barium. If oxygen
used as the reference, then Ca/Sr/Ba assigned atomic weights of 40, 88, and 137
respectively.
Rayleigh (physics) and Ramsey (chemistry) were awarded Nobel
prizes in 1904. The first inert gas compound was
made in 1962 (xenon tetrafluoride) and numerous compounds have followed (see
xenon compounds)--today the group is more appropriately called the noble
gases.
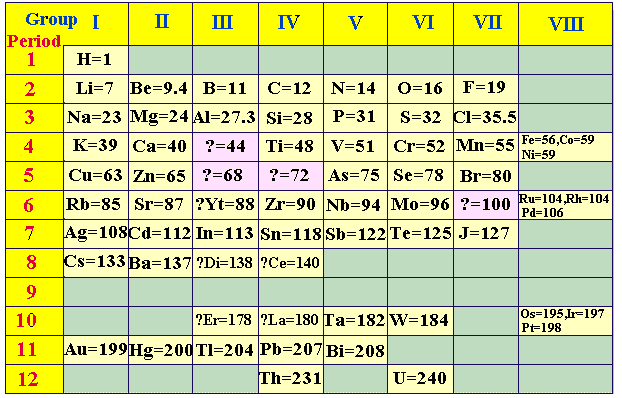
Moseley's Periodic Law
Soon after Rutherford's landmark experiment of discovering the
proton in 1911, Henry
Moseley (1887-1915) subjected known elements to x-rays.
He was able to derive the relationship between x-ray frequency and number of
protons. When Moseley arranged the elements according to increasing atomic
numbers and not atomic masses, some of the inconsistencies associated with
Mendeleev's table were eliminated. The modern periodic table is based on
Moseley's Periodic Law (atomic numbers). At age 28, Moseley was killed in action
during World War I and as a direct result Britain adopted the policy of
exempting scientists from fighting in wars. Shown
below is a periodic table from 1930:

Modern Periodic Table
The last major change to the periodic table resulted from Glenn
Seaborg's work in the middle of the 20th century. Starting with plutonium in
1940, Seaborg discovered transuranium
elements 94 to 102 and reconfigured the periodic table by placing the
lanthanide/actinide series at the bottom of the table. In 1951 Seaborg was
awarded the Nobel Prize in chemistry and element 106 was later named seaborgium
(Sg) in his honor.
Attention: New Additions to Periodic Table  
WOMANIUM (WO)
Physical properties: Generally soft and
round in form. Boils at nothing and may freeze any time. Very bitter if not used
well.
Chemical properties: Very active and highly
unstable. Possesses strong affinity with gold, silver, platinum, and precious
stones. Violent when left alone. Turns slightly green when placed next to a
better specimen.
Usage: An extremely good catalyst for
dispersion of wealth.
Caution: Highly explosive in inexperienced
hands!
MANIUM (XY)
Physical properties: Solid at room
temperature but gets bent out of shape easily. Difficult to find a pure sample.
Due to rust, aging samples are unable to conduct electricity as easily as young
samples.
Chemical properties: Attempts to bond with
WO any chance it can get. Also tends to form strong bonds with itself. Becomes
explosive when mixed with Childrium for prolonged period of time.
Usage: Possibly good methane source.
Caution: In the absence of WO,
this element rapidly decomposes and begins to smell.
 
- Periodic Table
-
Humor
Source of this page: http://mooni.fccj.org/~ethall/period/period.htm
Last modified
Contact us for more info

| 