|
Curriculum Guide for NCHS Chemistry By Objective
|
|
Goals
|
Content
|
Honors/Advanced
|
| COMPETENCY GOAL 1: The learner will build an understanding of the structure and properties of matter. (30% of curriculum) |
1.01 Summarize the development of current atomic theory.
(2%) |
Greeks - Democritus John Dalton's atomic theory.
J. J. Thomson - discovery of the electron
E. Rutherford gold foil experiment, nucleus
R. A. Millikan - charge on the electron
N. Bohr - Hydrogen spectrum and electron arrangement
(No names will be tested) |
|
1.02 Examine the nature of atomic structure:
1.021 Protons.
1.022 Neutrons.
1.023 Electrons.
1.023 Atomic mass.
1.024 Atomic number.
1.025 Electron configuration.
1.026 Energy levels.
1.027 Isotopes.
(4%) |
Properties of sub-atomic particles: relative mass, charge, and location in atom
Symbols: A and Z,
Principle quantum numbers; s, and p sublevels;
Electron configuraton (long and shorthand)
Orbitals notation using up/down arrows for opposite spin
Valence electrons
Lewis electron dot diagrams for atoms
Isotope notation: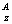 E (ie. E (ie.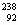 U or U-238) U or U-238)
Identify isotopes by mass and atomic number
|
Quantum numbers: azimuthal, magnetic and spin
Computation of energies and wavelengths in H spectrum.
|
1.03 Apply the language and symbols of chemistry
(4%) |
Binary nomenclature: Stock system for metal-nonmetal compounds and Greek prefix system for nonmetal-nonmetal compounds.
Stock system for compounds with polyatomic ions.
State symbols: (s), (l), (g)
Name the 6 strong acids and acetic.
Arrows indicating reactions and equilibria. |
Organic nomenclature, functional groups and named reactions. |
1.04 Identify substances using their physical properties:
1.041 Melting points.
1.042 Boiling points.
1.043 Density.
1.044 Color.
1.045 Solubility. (4%) |
Identify substances using their physical properties. Students should be able to read and apply information from reference tables. |
|
| 1.05 Analyze and explain the nature and behavior of the atomic nucleus including radioactive isotopes and their practical application. (4%) |
Characteristics of alpha, beta, gamma radiation: Relative masses, charges, symbols, penetrating ability; Shielding: air (alpha), metal (beta), and distance (qualitative use of inverse square law). Concepts of half-life, fission, and fusion.
Uses: dating, cancer therapy, smoke detectors, imaging. |
Decay equations.
Inverse square law calculations. |
1.06 Analyze the basic assumptions of kinetic molecular theory (KMT) and its applications:
1.061 Ideal Gas Equation.
1.062 Combined Gas Law.
1.063 Graham’s Law.
1.064 Dalton’s Law of Partial Pressures.
(4%) |
Five assumptions of KMT:
Avogadro's Law, PV=nRT, Boyle's Law, Charles' Law, P1V1/T1=P2V2/T2,
1 mole of any gas at STP = 22.4 L
Differentiate between real and ideal gases (factors nor calculations)
Graham's Law
Pt=P1+P2+ ... ; collecting a gas over water and vapor pressure of water.
Dalton's Law of Partial Pressures
|
Calculations of KE or speeds of molecules, Maxwell's distribution
Calculate molecular weight from effusion of gases
|
1.07 Assess the structure of compounds relating bonding and molecular geometry to chemical and physical properties;
1.071 Ionic bonds.
1.072 Covalent bonds.
1.073 Metallic bonds.
(3%) |
Electronegativity general trend - predict nature of bond.
Ion formation and stable arrangements (i.e. inert gas structure)
prediction of physical properties based on bonding (melting point etc.)
Lewis structures including single, double, triple bonds
VSEPR Theory:
Geometry: linear, bent, trigonal planar and tetrahedral, trigonal pyramidal.
Polar / nonpolar bonds, polar / nonpolar molecules and solubility in polar or nonpolar solvents. ("like dissolves like"). Include intermolecular forces to explain polarity. |
Resonance.
Formal charge calculations.
Geometries: trigonal bipyramidal, octahedral.
Hybrid orbital theory.
Molecular orbital theory.
|
| COMPETENCY GOAL 2: The learner will build an understanding of regularities in chemistry. ( 36% of curriculum) |
2.01 Analyze periodic nature of trends in chemical properties and examine the use of the Periodic Table to predict properties of elements;
2.011 Symbols.
2.012 Groups (families).
2.013 Periods.
2.014 Transition elements.
2.015 Ionization energy.
2.016 Atomic and ionic radii.
2.017 Electronegativity
(5%) |
Define family (group) and period.
Location on PT of alkali metals, alkaline earth metals, transition metals, rare earth metals, metalloids, halogens, inert gases. Also s, p, and d block elements.
General trends of electronegativity, and ionization energy.
Use PT to predict chemical and physical properties as well as charge of ions.
General trends in atomic and ionic radii,
Relate periodicity to electron configurations.
Students will always have PT to use. |
|
2.02 Analyze the mole concept and Avogadro's number and use them to calculate:
2.021 Mole to molecule.
2.022 Mass to moles.
2.023 Volume of a gas to moles.
2.024 Solution concentrations.
(5%)
|
Conversion factors using moles, mole-mole, mole-mass, mass-mass.
1 mole of any gas at STP = 22.4 L
Molarity.
Limiting factors, theoretical and actual yields.
Gravimetric and volumetric analysis.
Determine empirical and molecular formulas. |
Normality.
% concentration.
Molality.
|
2.03 Identify various types of chemical equations and balance those equations:
2.031 Single replacement.
2.032 Double replacement.
2.033 Decomposition.
2.034 Synthesis.
2.035 Combustion. (7%) |
Use references table on reaction types to identify reaction types and predict products.
Use activity series for single replacement.
Use solubility table and/or solubility rules for double replacement.
Write ionic and net ionic equations.
Arrhenius acid/base neutralization reactions. |
|
2.04 Calculate quantitative relationships in chemical reactions. (stoichiometry)
(7.5%) |
stoichiometry
mole-mole problems
mass-mass problems
mass-volume problems
volume-volume problems
gas laws and PV=nRT
molarity |
|
2.05 Identify the indicators of chemical change:
2.051 Formation of a precipitate.
2.052 Evolution of a gas.
2.053 Color change.
2.054 Absorption or release of heat. (4%) |
Recognize occurrence of reaction based on indicators of change such as formation of precipitate, evolution of a gas, color change and/or energy changes.
Use the solubility rules and activity series in reference materials to predict the outcome of reactions. |
|
2.06 Track the transfer of electrons in oxidation/reduction reactions and assign oxidation numbers:
2.061 Identify the oxidizing and reducing agents
2.062 Assess practical applications of oxidation and reduction reactions.
(4%) |
Using PT and ion chart, assign oxidation state for each element in a compound.
Show transfer of electrons by writing simple half reactions. Only simple metal/metallic ions will be tested.
Determine Voltage calculations.
Identify the element or ion oxidized, element or ion reduced, oxidizing agent, and reducing agent.
Know that redox reactions occur in batteries, combustion, corrosion, and electroplating. |
Redox equation balancing.
Primary vs secondary cells.
|
| COMPETENCY GOAL 3: The learner will build an understanding of energy changes in chemistry. (18% of curriculum) |
3.01 Observe and interpret changes (emission/absorption) in electron energies in the hydrogen atom including the quantized levels and their relationship to atomic spectra:
3.011 Electromagnetic radiation.
3.012 Light.
3.013 Photons. (3%) |
Hydrogen spectrum and Bohr model, electron transfer between "orbits" and relation to energy given off as light. Use reference table.
Use electromagnetic spectrum to relate wavelength and energy. Use equations only as illustration of relationship between energy and wavelength. c= fl, E=hf.
Particle and wave nature of light. |
No calculation of wavelength between two Bohr orbits. |
3.02 Analyze the law of conservation of energy, energy transformation, and various forms of energy involved in chemical reactions.
(5%) |
Connect to 3.04 - calorimetry, calculations of heat based on temperature change of a quantity of water, q=mcDT, definitions of enthalpy, exothermic, endothermic, heats of reaction and stoichiometry.
Energy vs pathway diagram showing energy of reactants, energy of products, enthalpy change, activation energy for exothermic and endothermic reactions.
Heating and cooling curves.
Phases Diagrams |
Hess's law, enthalpy calculations, heats of formation. |
3.03 Compare and contrast the nature of heat and temperature.
(4%) |
Temperature as measure of average kinetic energy of molecules.
Heat as q = mcDT, energy transferred from hot to cold.
Specific heat Specific heat. |
|
3.04 Analyze calorimetric measurement in simple systems and the energy involved in changes in state.
(5%) |
Calorimetry applications.
Heating curve for ice and or water showing plateaus at phase changes, include heat of fusion, vaporization for water. |
|
3.05 Analyze the relationship between energy transfer and disorder in the universe:
3.051 Nuclear.
3.052 Fossil fuels, Solar, Alternative sources.
(2%) |
General knowledge of how a nuclear reactor works.
Describe energy sources and the pros/cons of each energy source.
Definition of entropy and its implications. |
Calculations of entropy, enthalpy or Gibbs free energy. |
| COMPETENCY GOAL 4: The learner will build an understanding of equilibrium and kinetics. (16% of curriculum) |
4.01 Explain the dynamics of physical and chemical equilibria:
4.011 Phase changes.
4.012 Forward and reversible reactions.
(3%) |
Understand ice/water and water/vapor equilibrium.
Understand that some reactions don't go to completion, and an equilibrium is established. Write equilibrium express but no calculations.
Phase diagrams. |
Equilibrium expressions or Calculations.
Triple point.
|
4.02 Explain the factors that alter the equilibrium in a chemical reaction.
(4%) |
Le Chatelier's Principle - concentration, pressure, temperature.
Use the terms "shift to the right, shift to the left" or make more produce, make more reactant to describe changes.
Equilibrium expression as it relates to weak and strong acids but no calculations. |
Equilibrium expressions or calculations. |
4.03 Assess reaction rates and factors that affect reaction rates.
(4%) |
Rate as change in concentration (or pressure) as function of time. Factors affecting rate: concentration, pressure, temperature, catalyst (lower activation energy). |
Reaction order, time/concentration equations. Reaction mechanisms or rate determining steps. |
4.04 Compare and contrast the nature, behavior, concentration, and strength of acids and bases:
4.041 Acid-base neutralization.
4.042 Degree of dissociation or ionization.
4.043 Electrical conductivity.
4.044 pH.
(5%) |
Properties of acids and bases; Strength vs concentration; strength of weak acids and bases - partial dissociation.
Arrhenius and Bronsted.Lowery theories
Acid/base titration and stoichiometry. (nMV = nMV)
Weak Vs strong acids
pH scale and calculations with
pH=-log[H+], pOH=-log[OH-], pH + pOH = 14, [OH]= 10-pOH[H+]=10-pH
Buffer systems. (qualitative discussion only) |
Acid-base equilibria equations.
Lewis theory.
Ka and Kb calculations
|
