Gas Review Problem #1: GO BACK
1) A quantity of gas has a volume of 200 dm3
at 17oC and 106.6 kPa. To what temperature (oC) must
the gas be cooled for its volume to be reduced to 150 dm3 at a
pressure of 98.6 kPa?
Go
Back to Questions
Gas Review Problem #1
1) A quantity of gas has a volume of 200 dm3
at 17oC and 106.6 kPa. To what temperature (oC) must
the gas be cooled for its volume to be reduced to 150 dm3 at a
pressure of 98.6 kPa?
Write given information:
- V1 = 200
dm3
V2 = 150 dm3
- T1 = 17
oC
+ 273 = 290
K T2 =
_______
- P1 =
106.6 kPa
P2 = 96.8 kPa
Write equation:
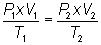
Substitute into
equation: 
Solve for T2:
T2 = 201 K
Recall:
oC + 273 = K
Therefore: Temperature
= -71oC
Last modified
Contact us for more info

| 