Gas Review Problem #2: GO BACK
2) A quantity of gas exerts a pressure of
98.6 kPa at a temperature of 22oC. If the volume remains
unchanged, what pressure will it exert at -8oC?
Go
Back to Questions
Gas Review Problem #2
2) A quantity of gas exerts a pressure of
98.6 kPa at a temperature of 22oC. If the volume remains
unchanged, what pressure will it exert at -8oC?
Write given information:
- V1 = constant
V2 = constant
- T1 = 22
oC
+ 273 = 295 K
T2 = -8 oC
+ 273 = 265 K
- P1 =
98.6 kPa
P2 = _________
Write equation:
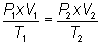
Volume is constant...cancel
it out from equation: 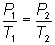
Substitute into equation: 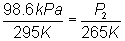 
Solve for P2:
P2 = 88.6
kPa
Last modified
Contact us for more info

| 