Gas Review Problem #4: GO BACK
4) What is the mass of
3.34 dm3 sample of chlorine gas if the volume was determined at 37oC
and 98.7 kPa? The density of chlorine gas at STP is 3.17 g/dm3.
Go
Back to Questions
Gas Review Problem #4
4) What is the mass of
3.34 dm3 sample of chlorine gas if the volume was determined at 37oC
and 98.7 kPa? The density of chlorine gas at STP is 3.17 g/dm3.
Write given information:
- V1 =
3.34 L
V2 = __________
- T1 = 37
oC
+ 273 = 310 K
T2 = 273 K
- P1 =
98.7
kPa
P2 = 101.3 kPa
- R = 8.314 kPa L /
mol
K
Density = 3.17 g/dm3
- n = ___________
- Cl2 = 71
g/mol
Two approaches to solve this
problem.
METHOD 1: Combined
Gas Law & Density
Write equation:
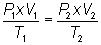
Substitute into
equation: 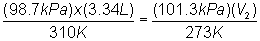
Solve for V2:
V2 = 2.85
L @ STP
Density = 3.17
g/dm3 @ STP
Recall: 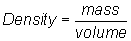
Substitute into
equation: 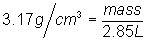 
Solve for
mass: mass = 9.1 g
chlorine gas
METHOD 2: Ideal
Gas Law
Write equation:
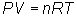
Solve for
moles:: 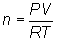
Substitute into
equation: 
Solve for mole: n
= 0.128 mol Cl2
Recall molar mass of diatomic
chlorine is 71 g/mol
Calculate mass of
chlorine: x g Cl2 = 0.128
mol Cl2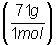 = 9.1 g Cl2
= 9.1 g Cl2
Last modified
Contact us for more info

| 