Gas Review Problem #5: GO BACK
5) In an airplane
flying from San Diego to Boston, the temperature and pressure inside the 5.544-m3
cockpit are 25oC and 94.2 kPa, respectively. How many moles of
air molecules are present?
Go
Back to Questions
Gas Review Problem #5
5) In an airplane
flying from San Diego to Boston, the temperature and pressure inside the 5.544-m3
cockpit are 25oC and 94.2 kPa, respectively. How many moles of
air molecules are present?
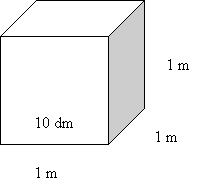
Convert
m3
to dm3:
x
dm3 = 5.544 m3
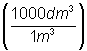 = 5544
dm3 = 5544
dm3
Write
given information:
- V = 5.544 m3
= 5544 dm3
- T = 25oC
+ 273 = 298 K
- P = 94.2
kPa
- R = 8.314 kPa L /
mol K
- n = ___________
Write equation:
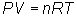
Solve for moles:
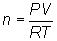
Substitute into
equation: 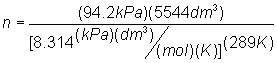 
Solve for mole: n
= 211 mol air
Last modified
Contact us for more info

| 