Gas Review Problem #6: GO BACK
6) Iron (II) sulfide reacts with hydrochloric acid
as follows:
FeS(s) + 2 HCl(aq)
--> FeCl2(aq) + H2S(g)
What volume of H2S, measured at 30oC
and 95.1 kPa, will be produced when 132 g of FeS reacts?
Go
Back to Questions
Gas Review Problem #6
6) Iron (II) sulfide reacts with hydrochloric acid
as follows:
FeS(s) + 2 HCl(aq)
--> FeCl2(aq) + H2S(g)
What volume of H2S, measured at 30oC
and 95.1 kPa, will be produced when 132 g of FeS reacts?
Calculate number of moles of
H2S...
x mole H2S = 132 g FeS 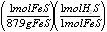 =
1.50 mol H2S =
1.50 mol H2S
Write given information:
-
P = 95.1
kPa
-
n = 1.5
mole H2S
-
R = 8.314
L kPa/mol K
-
T = 30oC
+ 273 = 303 K
Equation:
PV = nRT
Substitute into
Equation:
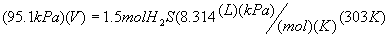 
Solve equation for
Volume: V = 39.7 L
Last modified
Contact us for more info

| 