Gas Review Problem #7: GO BACK
7) What is the density of nitrogen gas
at STP (in g/dm3 and kg/m3)?
Go
Back to Questions
Gas Review Problem #7
7) What is the density of nitrogen gas
at STP (in g/dm3 and kg/m3)?
Write given information:
1 mole N2 = 28 g N2
= 22.4 dm3 @ STP
Write equation:
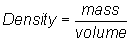
Substitute into
equation: 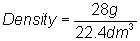
Solve for Density:
Density = 1.35 g/dm3
Recall: 1000
g = 1 kg & 1 m3
= 1000 dm3
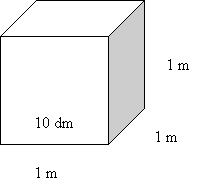
Convert
m3
to dm3:
x
dm3 = 1 m3
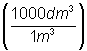 = 1000
dm3 = 1000
dm3
Convert:
 
Solve: 1.35
kg/m3
Last modified
Contact us for more info

| 