Gas Review Problem #8
8) A sample of gas at STP has a density
of 3.12 x 10-3 g/cm3. What will the density of the
gas be at room temperature (21oC) and 100.5 kPa?
Write given information:
- *V1 =
1.0 cm3 V2 = __________
- T1 =
273 K
T2 = 21oC + 273 = 294 K
- P1 =
101.3 kPa
P2 = 100.5 kPa
- Density = 3.17 g/dm3
*Density is an
INTENSIVE PROPERTY
Assume you
have a mass = 3.12 x 10-3 g
THEN: V1
= 1.0 cm3 [Recall Density =
3.12 x 10-3 g/cm3 ]
Write equation:
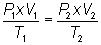
Substitute into
equation: 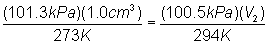
Solve for V2:
V2 = 1.0855 cm3
Recall:
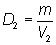
Substitute into
equation: 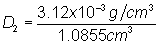

Solve for D2:
D2 = 2.87 x 10-3 g/cm3
