Gas Review Problem #9: GO BACK
9) Suppose you have a 1.00 dm3
container of oxygen gas at 202.6 kPa and a 2.00 dm3 container of
nitrogen gas at 101.3 kPa. If you transfer the oxygen to the container
holding the nitrogen,
a) what pressure would the nitrogen
exert?
b) what would be the total pressure
exerted by the mixture?
Go
Back to Questions
Gas Review Problem #9
9) Suppose you have a 1.00 dm3
container of oxygen gas at 202.6 kPa and a 2.00 dm3 container of
nitrogen gas at 101.3 kPa. If you transfer the oxygen to the container
holding the nitrogen,
a) what pressure would the nitrogen
exert?
b) what would be the total pressure
exerted by the mixture?
Write given information:
| |
Px |
Vx |
Vz |
Px,z |
|
O2 |
202.6 kPa |
1 dm3 |
2 dm3 |
101.3 kPa |
|
N2 |
101.3 kPa |
2 dm3 |
2 dm3 |
101.3
kPa |
|
O2
+ N2 |
|
|
2 dm3 |
202.6 kPa |
Part A: The
nitrogen gas would exert the same pressure (its partial pressure) independently
of other gases present
Write equation:

Pressure exerted by the
nitrogen gas = 101.3 kPa
Part B: Use
Dalton's Law of Partial Pressures to solve for the pressure exerted by the
mixture.
Write equation:
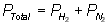
Substitute into
equation: 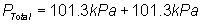 
Solve for PTotal
= 202.6 kPa
Last modified
Contact us for more info

| 