Neils Bohr:
GO BACK
 Ernest Rutherford's model
of the atom, developed at the turn of the century, pictured negatively charged
electrons moving in circular orbits about a positively charged nucleus.
Contradictory to electrodynamic theory the electrons did not emit
electromagnetic radiation. Niels Bohr provided the explanation by incorporating
Max Planck's quantum theory into Rutherford's atomic model. He envisioned
specific discrete energy levels (i.e., shells) for the electrons within which
they could move yet not emit radiation. Only if the electrons dropped to a lower
energy level, or were raised to a higher level, would they emit or absorb
electromagnetic radiation. That the energy of the emitted or absorbed radiation
must equal the difference between the original and final energy levels of the
electrons explained why atoms only absorb certain wavelengths of radiation. To
Albert Einstein, Bohr's achievements were "the highest form of musicality
in the sphere of thought". In recognition, Bohr received the Nobel Prize in
physics in 1922. Later, Louis de Broglie and Erwin Schrödinger described the
electron as a standing wave rather than a particle, which "explained"
how Bohr's electrons could move about within a defined energy level without
emitting radiation. This led Bohr to his famous principle of complementarity,
whereby the electron could be interpreted in two mutually exclusive yet equally
valid ways: by either the particle or wave models. Later, Bohr hypothesized how
an incoming particle could strike a nucleus and create an excited
"compound" nucleus. This idea formed the basis for his "liquid
drop" model of the nucleus which would provide Lise Meitner and Otto Frisch
with the theoretical basis for their explanation of fission. Ernest Rutherford's model
of the atom, developed at the turn of the century, pictured negatively charged
electrons moving in circular orbits about a positively charged nucleus.
Contradictory to electrodynamic theory the electrons did not emit
electromagnetic radiation. Niels Bohr provided the explanation by incorporating
Max Planck's quantum theory into Rutherford's atomic model. He envisioned
specific discrete energy levels (i.e., shells) for the electrons within which
they could move yet not emit radiation. Only if the electrons dropped to a lower
energy level, or were raised to a higher level, would they emit or absorb
electromagnetic radiation. That the energy of the emitted or absorbed radiation
must equal the difference between the original and final energy levels of the
electrons explained why atoms only absorb certain wavelengths of radiation. To
Albert Einstein, Bohr's achievements were "the highest form of musicality
in the sphere of thought". In recognition, Bohr received the Nobel Prize in
physics in 1922. Later, Louis de Broglie and Erwin Schrödinger described the
electron as a standing wave rather than a particle, which "explained"
how Bohr's electrons could move about within a defined energy level without
emitting radiation. This led Bohr to his famous principle of complementarity,
whereby the electron could be interpreted in two mutually exclusive yet equally
valid ways: by either the particle or wave models. Later, Bohr hypothesized how
an incoming particle could strike a nucleus and create an excited
"compound" nucleus. This idea formed the basis for his "liquid
drop" model of the nucleus which would provide Lise Meitner and Otto Frisch
with the theoretical basis for their explanation of fission.
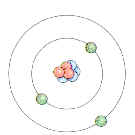 The
Bohr Model of the Atom (The Planetary Model) The
Bohr Model of the Atom (The Planetary Model)
Neils
Bohr
Amedeo Avagadro
Antoine Lavoisier
William Proust
J.J.
Thomson
Sir William Crookes
Albert
Einstein
Ernest Rutheford
Max
Planck
John
Dalton
Dimitri Mendeleev
Hans Geiger
Jeff Christopherson
Democritus of Abdera, c. 450 BCE
Additional Information
Last modified
Contact us for more info

|

|
