Nuclear Chemistry
PowerPoint
*V I D E O L E S S O N S
*Corwin Textbook - Publisher Website with Objectives and Quizzes
*Corwin Textbook questions pdf
*Vocabulary pdf
*Nuclear Equations pdf
*Half-Life pdf
*Nuclear Energy Transformations pdf
*Back to Chernobyl video guide pdf
RADIATION Fact Sheet
A) Electromagnetic Radiation
- light (range of frequencies: radio waves to g-rays)
- travels at speed of light (3 x 108 m/s)
- streams of massless particles (photons)
Photons effect depends on their energy, which increases as the wavelength is shorter (higher frequency)
c = f l E = h f
Amount of radiation depends on the intensity (i.e. the number of photons).
Example: You can get a sunburn in 20 minutes  or spend weeks behind glass (which cuts out the UV, but lets through the visible light) without a tan. or spend weeks behind glass (which cuts out the UV, but lets through the visible light) without a tan.
B) Streams of Particles (with rest mass traveling at various speeds) 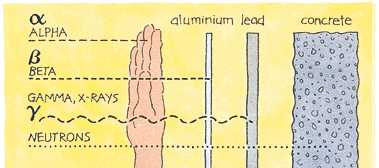
C) Radioactive Disintegration
- half-life table
- band of stability
- activity - rate at which radioactive nuclei emits particles
- one Becquerel (Bq) is 1 radioactive disintegration / second
- strong force [aka binding energy: holds nucleus together - recall protons want to repel each other]
D) Biological Effect
- Many factors to consider...
- Analogy: If I punch you in the nose hard - I transmit energy from my fist to your nose. A hard punch can draw blood. The amount depends on how often I repeat the process. A soft blow has little effect, no matter how often it is repeated. In fact, a whole series of soft blows may far exceed in energy, the energy of a single hard blow, but is not absorbed in a manner that causes damage.
- Absorb UV vs. Visible sunlight
E) External Exposure to Radiation
- U-238 (granite)
-
 (clay) (clay)
- cosmic rays (10 trans-Atlantic plane tripe = whole year at sea level)
- radon gas - from decay of radium
- radiation safety
F) Art Forgery Detection & Other Crime Detection
G) Food Irradiation
- 1950's Eisenhower "Atoms for Peace" initiative
H) Medical Treatments
- PET scans
- chemotherapy
- X-rays (radiology)
- Nuclear medicine
- Magnetic Resonance Imaging (MRI)
- Gamma knife (surgery)
I) Other Uses of Radiation in Everyday Life
J) Nuclear Accidents
K) Atomic Bomb - Factoids
Nuclear Chemistry deals with the nuclei of atoms breaking apart. Atoms are continually undergoing decay. When studying nuclear chemistry, there is a typical format used to represent specific isotopes.
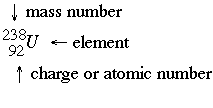
Nuclear equations are typically written in the format shown below. There are 5 different types of radioactive decay.
- Alpha decay follows the form:
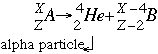
Where A is the parent isotope (the atom being broken apart) B is the daughter isotope or the isotope formed. When an element is broken down in alpha decay it looses two neutrons and two (2) protons. This means that the name of the element will change as well, moving back two (2) places on the periodic table. Alpha decay is is not very penetrating because the He atoms capture electrons before traveling very far. However it is very damaging because the alpha particles can knock atoms off of molecules. Alpha decay is the most common in elements with an atomic number greater than 83.
- Beta negative decay follows the form:
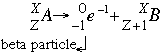
The beta emission increases the atomic number by one (1) by adding one (1) proton. At the same time, one (1) neutron is lost so the mass of the daughter isotope is the same as the parent isotope. Beta negative decay is more penetrating than alpha decay because the particles are smaller, but less penetrating than gamma decay. Beta electrons can penetrate through about one (1) cm of flesh before they are brought to a halt because of electrostatic forces. Beta decay is most common in elements with a high neutron to proton ratio.
- Gamma decay follows the form:
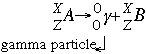
In gamma emission, neither the atomic number or the mass number is changed. A very highly charged gamma ray is given off when the parent isotope falls into a lower energy state. Gamma radiation is the most penetrating of all. These photons can pass through the body and cause damage by ionizing all the molecules in their way.
- Positron emission (also called Beta positive decay) follows the form:
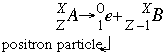
In this reaction a positron is emitted. A positron is exactly like an electron in mass and charge force except with a positive charge. It is formed when a proton breaks into a neutron with mass and no charge and this positron with no mass and the positive charge. Positron emission is most common in lighter elements with a low neutron to proton ratio.
- Electron capture follows the form:
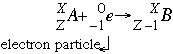
In this reaction a nucleus captures one (1) of its own atom's inner shell electrons which reduces the atomic number by one. This captured electron joins with a proton in the nucleus to form a neutron. Electron capture is common in larger elements with a low neutron to proton ratio.
All elements with an atomic number over 83 are considered radioactive. Radioactivity can be measured using a geiger counter, a cylinder containing a low-pressure gas and two (2) electrodes. Radiation ionizes the atoms in the cylinder and allows current to flow between the electrodes.
All radioactive elements disintegrate according to their specific half life. The half life of a radioactive substance is the time required for half of the initial number of nuclei to disintegrate. The decay rate expresses the speed at which a substance disintegrates. The following equation represents the relationship between the number of nuclei remaining, N, the number of nuclei initially present, NO, the rate of decay, k, and the amount of time, t.
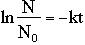
The relationship between the half-life of a radioactive substance and k, the rate at which it decays can also be found.
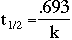
By using these equations, it is possible to calculate how much of a nuclear substance will be left after a certain time and how much of a substance originally existed. A common example is isotopic dating in which the ages of archeological artifacts are determined by measuring the activity of the isotopes. The variable n is the number of half-lifes.
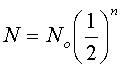 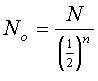
Practice Problems
1. The half life of a specific element was calculated to be 5200 years. Calculate the decay constant (k). Solution.
2. If a watch contains a radioactive substance with a decay rate of 1.40 * 10 -2 and after 50 years only 25 mg remain, calculate the amount originally present. Solution.
3. A rock contains 0.257 mg of lead-206 for every mg of uranium-238. The half-life decay for uranium to turn into lead is 4.5x109 yr. How old is the rock? Solution.
Breeding Pu-239 from U-238
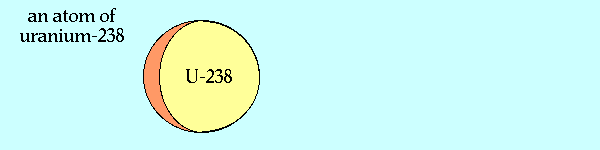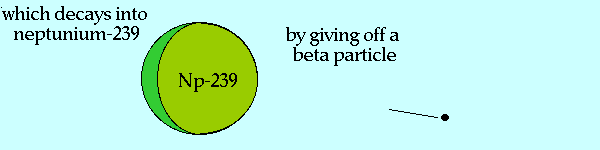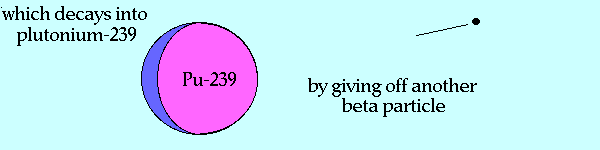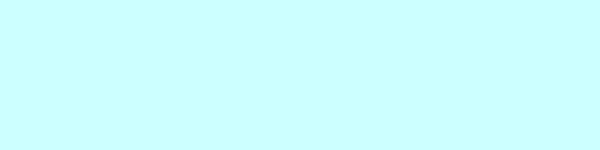 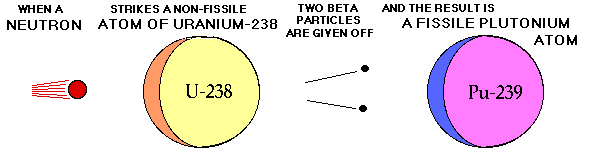
Source of graphic: http://www.ccnr.org/breeding_ana.html
[Nuclear Fission ]
A Nuclear Calculator and a Disintegration Time Calculator are available for use.
Radiation Topics Plutonium Properties
Nuclear Energy Timeline PowerPoint
Useful Background Animated Wavefronts
Famous Nuclear Scientists Vocabulary HW pdf
ChemCases.com CERN Glow Ring
Help Files for Nuclear Chemistry
Nuclear Experiments Cs-137 EPA sight Radiation Fact Sheet
Glossary of Nuclear Terms Glossary
ABC's of Nuclear Science
The Living Textbook of Nuclear Chemistry
The Pros and Cons of Nuclear Energy
Nuclear Chemistry - Tutorials
Nuclear - New Zealand
How Nuclear Radiation Works
Alpha Radiation in Lung Tissue of Ape
Inertial Fusion Energy
Government Agency Web sites
Topics List
Last modified

|
