J.J. Thomson:
GO BACK
In
1884, at age 28, J.J. Thomson became Director of the Cavendish Laboratory at
Cambridge University.
No one was more surprised than Thomson who had been
decried as a "mere boy". Nevertheless, this mere boy turned what he
described as a "string and sealing wax laboratory" into the world's
preeminent center for experimental nuclear physics. It has been said that
Thomson, like Michael Faraday, was greater than his discoveries. However,
those discoveries were far from insignificant. Thomson and his student
Ernest Rutherford were the first to demonstrate the ionization of air by X
rays. So fundamental is this phenomenon that the phrase "ionizing radiation" remains the most concise way to characterize the wide range of
electromagnetic and particulate radiation emitted by atoms. Nevertheless,
Thomson is best known for his investigations into the nature of "cathode
rays", (i.e., electrons). By deflecting these "rays" with an electric field,
something that had been done previously with a magnetic field, Thomson
provided conclusive proof that they were negatively charged particles. He
determined their mass to be one two-thousandth that of the hydrogen atom,
the smallest object known at that time. Thomson was thus the first to
identify the existence of subatomic particles. This opened the door to a new
world of which his student, Ernest Rutherford, would later master, as well
as provide his own significant contributions to nuclear physics. Later,
Thomson demonstrated that the interaction between electrons and matter
produced X rays and that X rays interacting with matter produced electrons.
Although it would fail the test of time, Thomson is usually credited with
the first "modern" model of the atom, the so-called "plum pudding" model. In
it, he pictured a sphere of positive charges mixed together with an equal
number of electrons (i.e., negative charges). For his theoretical and
experimental investigations into the electron and the conduction of
electricity by gases, Thomson was awarded the 1906 Nobel Prize in physics.
Ironically, Thomson, who had characterized the material properties of
electrons, would live to see his son George P. Thomson receive the Nobel
Prize for experimentally confirming the wavelike properties
of
electrons. one was more surprised than Thomson who had been
decried as a "mere boy". Nevertheless, this mere boy turned what he
described as a "string and sealing wax laboratory" into the world's
preeminent center for experimental nuclear physics. It has been said that
Thomson, like Michael Faraday, was greater than his discoveries. However,
those discoveries were far from insignificant. Thomson and his student
Ernest Rutherford were the first to demonstrate the ionization of air by X
rays. So fundamental is this phenomenon that the phrase "ionizing radiation" remains the most concise way to characterize the wide range of
electromagnetic and particulate radiation emitted by atoms. Nevertheless,
Thomson is best known for his investigations into the nature of "cathode
rays", (i.e., electrons). By deflecting these "rays" with an electric field,
something that had been done previously with a magnetic field, Thomson
provided conclusive proof that they were negatively charged particles. He
determined their mass to be one two-thousandth that of the hydrogen atom,
the smallest object known at that time. Thomson was thus the first to
identify the existence of subatomic particles. This opened the door to a new
world of which his student, Ernest Rutherford, would later master, as well
as provide his own significant contributions to nuclear physics. Later,
Thomson demonstrated that the interaction between electrons and matter
produced X rays and that X rays interacting with matter produced electrons.
Although it would fail the test of time, Thomson is usually credited with
the first "modern" model of the atom, the so-called "plum pudding" model. In
it, he pictured a sphere of positive charges mixed together with an equal
number of electrons (i.e., negative charges). For his theoretical and
experimental investigations into the electron and the conduction of
electricity by gases, Thomson was awarded the 1906 Nobel Prize in physics.
Ironically, Thomson, who had characterized the material properties of
electrons, would live to see his son George P. Thomson receive the Nobel
Prize for experimentally confirming the wavelike properties
of
electrons.
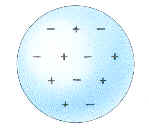
Thomson's 'plum-pudding' model of the atom.
Neils
Bohr
Amedeo Avagadro
Antoine Lavoisier
William Proust
J.J.
Thomson
Sir William Crookes
Albert
Einstein
Ernest Rutheford
Max
Planck
John
Dalton
Dimitri Mendeleev
Hans Geiger
Jeff Christopherson
Democritus of Abdera, c. 450 BCE
Additional Information
Last modified
Contact us for more info

|

|
