Gas Law Calculations:
Boyle's Law
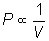 or
or 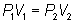 Boyles Law Cartoon
Boyles Law Cartoon

(Boyle's law calculator)
Balloon in bell jar (shaving
creme)
Charles' Law
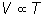 or
or 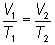 Balloon in Liquid Nitrogen*
Balloon in Liquid Nitrogen*
(Charles' law
calculator)
Gay-Lussac's
Law 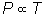 or
or 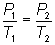
(Gay-Lussac's law
calculator)
Combined Gas Law
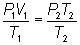
(Combined gas law
calculator)
Graham's Law of Diffusion
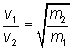 vs. Effusion
Needle through a balloon
vs. Effusion
Needle through a balloon
Dalton's Law of Partial Pressures
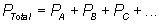
Ideal Gas Equation
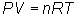
Gas constant,
R
R = 0.0821 atm L / mol K or R = 8.31 kPa L /
mol K
PowerPoint
II
Collect
A Gas With A Pneumatic Trough
Vapor Pressures of Water at Selected
Temperatures
Last modified
Contact us for more info

| 